Enough to make you sick

| Fake medicines are concoctions with wrong ingredients or incorrect quantities
of active ingredients or products without any active ingredients at all |
| It is virtually impossible to differentiate between real and counterfeit
medicines, given the current institutional capacity |
| Nearly 10 per cent of the global pharmaceutical commerce is attributed to fake
drugs |
| Combating this menace should be a shared responsibility |
Devinder Singh of Patiala is an angry man. His 14-year old son
Amarpreet, suffers from Wilson's Disease, which was diagnosed in 1997. Prior to this,
Amarpreet was an active boy who loved playing football. Wilson's disease is a genetic
disorder wherein the intestines absorb more copper and accumulate it in the liver. The
accumulation of copper causes its release directly into the bloodstream, which poisonsthe
whole body. This damages the kidneys, brain, and eyes and ultimately causes death.
The first symptom that occurred in Amarpreet’s case was cramping
of fingers. That was followed by loss in speech and problem in walking, eventually
resulting into paralysis. On diagnosis, Penicillamine capsules were prescribed (that were
marketed by Biochemie Austria) by Christian Medical College (CMC) Hospital in Vellore.
Amarpreet recovered completely and he resumed his usual activities right from walking,
normal speech and even attending school. However, a follow-up visit to the doctor turned
into a nightmare for the family.
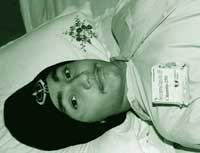
DEVINDER SINGH |
Paralysed
Amarpreet Singh |
In June 1999, Amarpreet was prescribed
Cilamin 250 capsule, the Indian Penicillamine capsule manufactured and marketed by M/s
Panacea Biotec Ltd. The medicine was provided by the CMC pharmacy. The quality of the
medicine was never questioned, nor did it occurto anybody that the medicine would cause a
relapse of the disease. It was only when Amarpreet's condition started deteriorating after
taking these medicines, and he was taken to CMC again, that the doctors questioned the
authenticity of the medicines given (see photo: Amarpreet Singh).
 |
 |
It took Devinder Singh two long years
merely to get the medicine tested and prove its substandard quality. He was turned down by
a number of reputed laboratories. Société Générale de Surveillance (SGS) India (part
of the globally reputed chain of testing laboratory) conducted this test only after Singh
contacted the SGS Geneva office. A certificate of analysis from SGS India in Chennai dated
February 4, 2002 clearly indicates the substandard quality of the medicine (see:
Certificates of analysis issued by SGS).
During this long struggle of pleading in
courts and demanding justice, Amarpreet has already crossed the two-year "window
period" for recovery from the disease. Devinder Singh's case has now been accepted as
a public interest litigation case and hearings have commenced. In a country where human
life has minimal value, Devinder's two-year struggle for justice is no surprise. The
laboratories made every attempt to delay the tests and the results. Reporting of
grievances to the Drug Controller and the Chief Vigilance Officer led nowhere.
Singh researched and managed to get the
support of two more patients suffering from Wilson's disease, where spurious drugs caused
further deterioration of their health. One was the case of S P Arora's son, Rajesh Arora,
from Rajpura in Punjab, who at the age of 32 years old, weighed only 30 kilogrammes and
had his spinal cord curled up. He would easily slip underneath the space between the front
and back seats of a Maruti 800 (see photograph: Rajesh Arora, p10). Rajesh died in
July 2002. In Patiala, Anoop Singh's son who too suffered from Wilson's disease died in
1998 due to consumption of the Cilamin capsules.
Several such cases occur every day in
India. Many go unreported and unnoticed.
Fundamentals of counterfeiting drugs Counterfeit drugs or
fake medicines are concoctions with wrong ingredients or incorrect quantities of active
ingredients or products without any active ingredients at all. Products with incorrect
quantities of ingredients or expired medicines may initiate allergic reactions in patients
and cause harmful interactions with other administered drugs. Products with the wrong
ingredients are toxic and harmful, often causing death. A review by the World Health
Organisation (WHO) titled "Global trade in counterfeit drugs," found that 60 per
cent of fake drugs had no active ingredients, 16 per cent had the incorrect ingredients
and 17 per cent had the incorrect amount.1
What makes the case of counterfeit drugs so
dangerous is that it is virtually impossible to differentiate between real and counterfeit
medicines. J N Pande, head of medicine, All India Institute of Medical Sciences (AIIMS),
New Delhi says, "The most worrying aspect is that the majority of the fake drugs are
in the fast moving category. They may contain the same salts (as the original) but the
purity and quantity is lower. As a result, patients consume more drugs to get
results." Since doctors, patients, and other medical staff fail to recognise fake
medicines, they do not doubt the authenticity of the pills and tablets, and when these
medicines fail to cure, some other causes are held responsible. According to Ravi Kant,
Assistant Drug Controller of Delhi, "People have begun to use good quality paper and
dyes. The foils on tablets and the paper wrapping used for packaging, all look well made.
All in all there is no give away on this stuff.

